HCOOH CH2 H2O, most often identified with the formic acid (HCOOH) and its complexes with the molecules of methylene (CH₂) and water (H₂O) is a wonderful example of a chemical system, having both peculiarities of the structure and functions.
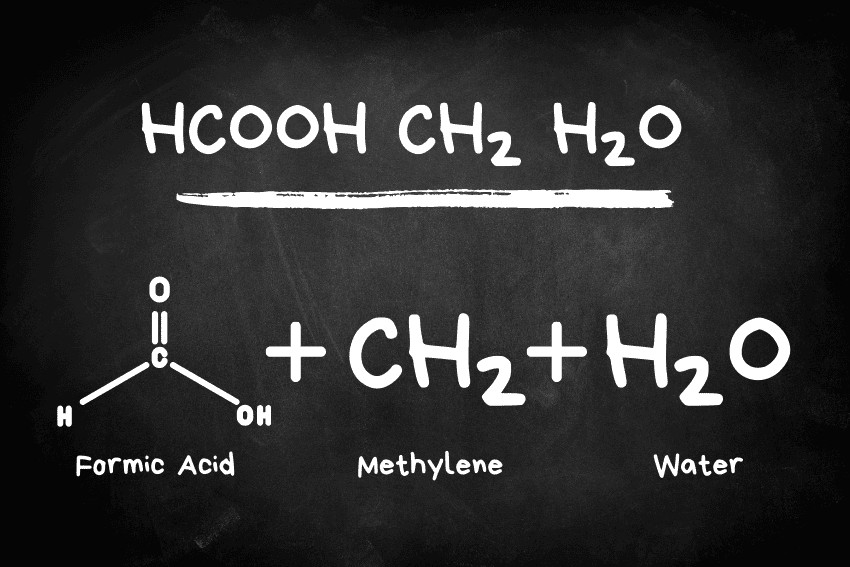
This article explores in detail the molecular composition, physical and chemical properties and real-world uses of this system and makes it interesting to students, researchers and industry players.
Exploring HCOOH CH₂ H₂O in a more scientific light, we intend to deliver a well-calculated and easy-understandable source in the best interest of human readers as well as those with AI-driven search engines.
Understanding the Components of HCOOH CH2 H2O
To understand the meaning behind HCOOH CH₂ H₂O, we will first need to decompose it:
- Formic Acid (HCOOH): Formic acid; also called methanoic acid, is the simplest carboxylic acid. It is a hydrogen-bonded carboxyl group (COOH). It can be found in natural conditions in the stings of ant (and other insects) and in some plants, but it is also utilized in industrial and biological applications.
- Methylene (CH₂): Methylene is a very reactive carbene molecule that contains a divalent carbon atom with two hydrogen atoms bound to it. It is a short-lived form, found fleetingly in chemical reactions.
- Water (H₂O): Water is a universal solvent and forms an essential part of chemical bonding interaction, acting either as a reagent or a stabilizing medium.
Together, HCOOH CH2 H2O is not actually a specific compound one is likely to encounter but is used as an example in benign ways, mainly in theoretical studies or when creating a reaction.
Molecular Structure and Bonding
The structure of HCOOH CH2 H2O as a system depends on the context of their interaction. HCOOH or Formic acid is a planar molecule, and the carbon has one hydroxyl group (OH), carbonyl (C=O) and hydrogen. It is a strong polar molecule that has the capacity to form hydrogen bonds, and it is therefore water-soluble because water too is a polar molecule with a bent shape that allows it to form hydrogen bonds.
As a carbene, the unstable Methylene (CH2) is very reactive because it has an incomplete octet. It can be involved in temporary contact between HCOOH and H2O, including insertion actions or binding with the oxygen molecules of water. Computational investigations of chemical reactions often polarize these interactions to learn about reaction pathways, such as those found in atmospheric chemistry or catalysis.
A combination of HCOOH CH2 H2O is usually investigated in gas-phase reactions or in water where the hydrogen bonding and the van der Waal forces are predominant. An example is whereby water could stabilize the reactive intermediate such as CH2 whereas formic acid is acidic, and this alters the reactivity of the total system.
Physical and Chemical Properties
The properties of the HCOOH CH2 H2O system are determined by its constituents and their interactions:
Formic Acid (HCOOH):
- Physical State: Colorless liquid having a pungent smell in room temperature conditions.
- Boiling Point:8°C (213.4°F), near that of water, because of intense hydrogen bonding.
- Solubility: Dissolvable in water giving it a bigger part in reactions which occur in water.
- Acidity: pKa of 3.75, a stronger acid than acetic acid therefore it is suitable to donate protons in reactions.
- Reactivity: Reacts as a reducing and an oxidizing agent, decomposing to CO2 and H2 under some circumstances.
Methylene (CH₂):
- Reactivity: Highly reactive owing to its singlet or triplet electronic states, and they can take part in addition or insertion reactions.
- Stability: Unstable, easily requiring stabilization by solvents such as water or coordination complex with other molecules.
Water (H₂O):
- Polarity: Facilitates solvation and hydrogen bonding, critical for stabilizing HCOOH and CH2.
- Dielectric Constant: High (78.5 at 25 oC), enabling it to support ionic and polar interactions.
The combination of the HCOOH CH2 H2O system is dynamic. As an illustration, the acidity of formic acid can cause the pH of an aqueous solution to decrease, and because methylene is reactive, there may be transient adducts or reaction intermediates. These features make the system pertinent in other areas such as organic synthesis and environmental chemistry.
Applications of HCOOH CH2 H2O
The research of HCOOH CH2 H2O is applied in various fields, including industrial and science research. These are some of the main areas of interest where this system functions in:
Organic Synthesis
In organic chemistry HCOOH interactions with CH2 H2O are studied in reaction mechanisms of carbenes. In situ, methylene can be combined with formic acid in aqueous conditions, and eventually produce intermediates towards the production of alcohols, ethers, or other organic compounds. Water is a solvent that stabilizes reactive species and participates in the transfer of protons.
Environmental Chemistry
Formic acid: This is one of the main volatile organic compounds in the atmosphere that causes acidity of rain waters. The system HCOOH CH2 H2O has also been modeled to investigate how, in atmospheric chemistry, carbenes such as CH2 formed through a photochemical reaction interact with water vapor and organic acids. This information assists in forecasting the behavior of pollutants and atmospheric degradation pathways.
Catalysis
Formic acid is a potential hydrogen storage material that liberates H2 and CO2 when dissociated with catalysts. The HCOOH CH2 H2O system is of interest in catalytic work in which water participates in reaction rates, and the formation of methylene-like species can contribute to gas-phase decomposition. This is vital in coming up with sustainable energy solutions.
Biomedical Research
The presence of Formic acid in biological systems, e.g. ant venom, sees it as an object to study in terms of biochemical processes. The system of HCOOH CH2 H2O has been studied computationally to find out how reactive species such as methylene can react with aqueous biological systems, which may be relevant to drug design or toxicology.
Material Science
Reducing behavior of Formic acid is also applied in the synthesis of nanomaterials, and the medium is water. The HCOOH CH2 H2O system may be of interest when examining the impact of transient species such as CH2 on the development and formation of nanoparticles or surface coating.
Why HCOOH CH2 H2O Matters
The system HCOOH CH2 H2O is not just a theory with no application to the real world. The research enhances our knowledge of molecular interactions and reactions, environmental processes. To scientists, it provides an arena to conduct research on reactive intermediates and their stabilization. The industries will also enjoy an understanding of the feasible processes, such as hydrogen generation to control pollution.
Future Directions
As computational chemistry becomes more sophisticated the HCOOH CH2 H2O system is expected to be resolved with much greater accuracy identifying new reaction paths. Breakthroughs in catalysis and green chemistry have the potential to exploit the properties of formic acid, and water could be critical to large-scale applications. Monitoring the environment will also benefit through knowing how these molecules interact in nature itself, benefiting our stewardship of the environment by helping to deal with climate change.
Conclusion
HCOOH CH2 H2O summarizes a dynamic chemical process that has tremendous consequences. This system is appreciated in terms of its molecular complexity, as well as its many uses in synthesis, catalysis, environmental science and more, demonstrating the beauty of chemistry in a practical dilemma solution. By researching HCOOH CH2 H2O we open the doors to solutions of the problems with molecules behavior and the future innovations in science and industry.
FAQs
What is HCOOH CH2 H2O commonly known as?
It can be simply defined as a hydrate of formic acid containing a methylene molecule and is usually the subject of research in areas such as hydrogen bonding and solvation.
Is HCOOH CH2 H2O the same as formic acid?
Not exactly. Although the structures of formic acid (HCOOH) and HCOOH CH2 H2O are close, the second molecule is more hydrated and includes an extra compound, which adds some differences to the molecule and its properties.
Where is HCOOH CH2 H2O used most?
It is mainly applied on research, chemical synthesis and in atmospheric chemistry studies, although some potential industrial applications are there.